Jones Reagent
(CrO3 in H2SO4)
General Information:
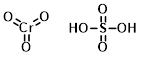
CAS Number: 65272-70-0
Density: 1.298 g/mL (2M CrO3 in aq H2SO4)
Jones Reagent is mixture of chromium trioxide in sulfuric acid which is used for Jones oxidation, typically with acetone as the solvent. Jones oxidation reactions are not commonly used anymore due to the toxicity of chromium (VI) reagents.
Common Uses:
Reagent for the conversion of primary alcohols to carboxylic acid (Jones oxidation)
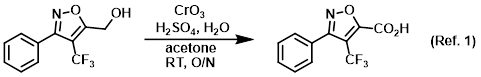
Procedure excerpt:
To an orange, homogeneous solution of CrO3 (12.4 g, 0.123 mol) in H2O (88.4 mL) at 0 C was added H2SO4 (10.8 mL) dropwise via addition funnel over 30 min, with stirring. . . .
Safety:
Jones reagent is highly toxic.
References:
1) Patent Reference: WO2011017578, page 69, ![]() (8.3 MB)
(8.3 MB)
2) Tojo, G.; Fernandez, M.; Oxidation of Primary Alcohols to Carboxylic Acids
3) Wikipedia: Jones oxidation (link)
4) www.sigmaaldrich.com: Jones reagent (link)
